What Are Hydrogen Fuel Cells and How Do They Work? Pros and Cons Explained
Picture vehicles and devices generating electricity with water vapor as their only emission. This tangible potential is driving massive investment, reflected in the global hydrogen fuel cell market – estimated at USD 4.35 billion in 2024 and projected to surge to around USD 27.49 billion by 2034, expanding at a CAGR of 20.24%[1].
As industries and governments explore alternatives to fossil fuels and batteries, understanding this technology becomes essential. How do fuel cells convert hydrogen into electrical power? What genuine advantages do they offer, and what hurdles must they overcome? This guide provides a clear explanation of hydrogen fuel cell operation; keep reading!

How Does a Hydrogen Fuel Cell Work?
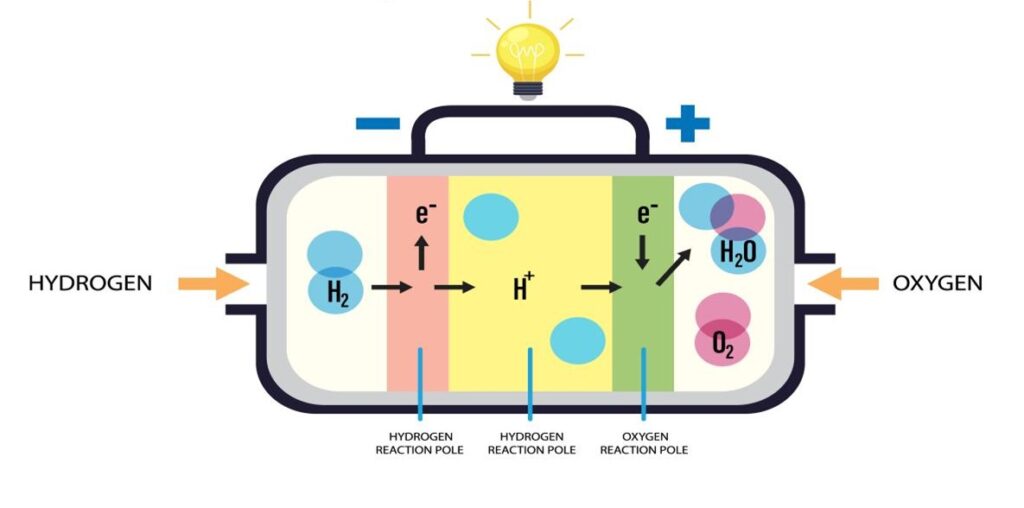
Hydrogen fuel cells generate electricity through an electrochemical reaction between hydrogen and oxygen, producing only water and heat. Here’s the process:
Splitting Hydrogen at the Anode: Hydrogen gas (H2) enters the fuel cell and flows over the anode. A catalyst coating on the anode helps split each hydrogen molecule (H2) into two positively charged hydrogen ions (protons, H+) and two negatively charged electrons (e–).
H2 → 2H⁺ + 2e⁻
Generating Current & Proton Journey: The freed electrons cannot pass through the core component separating the electrodes. Instead, they are forced to travel through an external electrical circuit. Meanwhile, the positively charged protons (H+) take a different path. They move through a specialized, selectively permeable material called the Proton Exchange Membrane (PEM) (or electrolyte). This membrane allows only protons to pass directly from the anode side to the cathode side.
Forming Water at the Cathode: On the other side of the cell, oxygen gas (O2) flows over the cathode. Here, aided by another catalyst, the incoming protons (H+) that passed through the membrane, the electrons (e–) that completed their journey through the external circuit, and the oxygen molecules combine. This reaction forms pure water (H2O) as the primary byproduct, which is expelled, along with some heat.
4H+ + 4e– + O2 → 2H2O
Advantages of Hydrogen Fuel Cells
Hydrogen fuel cells offer several compelling benefits driving their development:
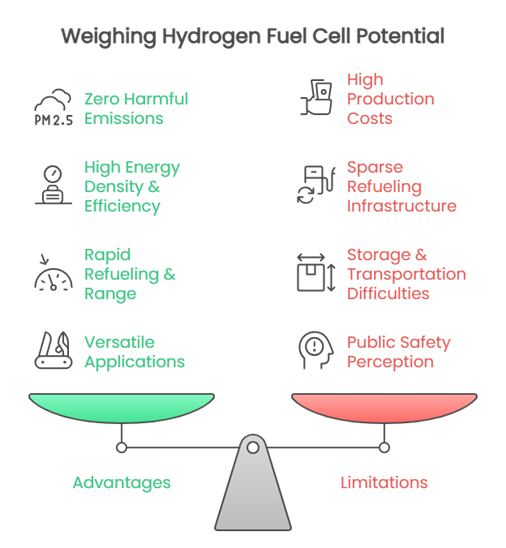
Zero Harmful Emissions
The core electrochemical reaction produces only water vapor and heat as direct byproducts. When using “green hydrogen” (produced via renewable energy), the entire lifecycle can be virtually emission-free, significantly reducing local air pollution and greenhouse gases compared to fossil fuels.
High Energy Density & Efficiency
Hydrogen gas stores a large amount of energy per unit of weight, contributing to impressive hydrogen fuel cell efficiency. This translates to potentially longer ranges for vehicles compared to battery-electric equivalents of similar weight. Fuel cells also convert chemical energy directly to electricity with higher efficiency than traditional combustion engines.
Rapid Refueling & Range
Refilling a hydrogen tank takes minutes, similar to gasoline or diesel, addressing the longer charging times associated with battery electric vehicles. This, combined with high energy density, enables long driving ranges ideal for heavy-duty transport.
Versatile Applications
Their characteristics make them particularly suitable for demanding uses: long-haul trucks, buses, trains, industrial machinery requiring continuous operation, and critical backup power systems where downtime is unacceptable.
Limitations and Challenges
Despite their potential, hydrogen fuel cells face significant hurdles:
High Production Costs
Producing hydrogen, especially “green hydrogen” via electrolysis using renewable electricity, is currently often more expensive than conventional fuels or direct battery charging. This high cost impacts the overall viability of fuel cell systems.
Sparse Refueling Infrastructure
The network of hydrogen refueling stations is extremely limited compared to gasoline stations or electric vehicle chargers. Building this infrastructure requires massive investment, slowing down adoption, particularly for consumer vehicles.
Storage & Transportation Difficulties
Hydrogen is the lightest element and has very low energy density by volume at ambient conditions. Storing and transporting sufficient quantities for practical use typically requires expensive high-pressure tanks, complex cryogenic systems, or advanced materials, adding complexity and cost.
Public Safety Perception
Although hydrogen fuel cells are designed with rigorous safety standards, public concerns persist due to hydrogen’s flammability over a wide range of concentrations in air and its invisible flame. Effective handling requires specific protocols and public education.

Join Mobility Tech Asia 2025 to Explore the Future of Hydrogen Energy
To explore the future of hydrogen, join global hydrogen leaders, pioneering researchers, and forward-thinking investors at the Hydrogen Summit, part of Mobility Tech Asia 2025, from 15–17 July 2025 at AsiaWorld-Expo, Hong Kong.
Under the theme “Shaping the Future of the Global Hydrogen Industry”, this summit will delve into market development trends, innovative technologies, and international cooperation within the hydrogen sector.
Register now to engage with over 50 world-class exhibitors showcasing advancements in fuel cell systems, hydrogen storage, and mobility applications.
Reference
[1] Hydrogen Fuel Cells Market Size, Share, and Trends 2025 to 2034. Available at: https://www.precedenceresearch.com/hydrogen-fuel-cells-market (Accessed: 29th, May)

